Guidance for Industry
|
|
|
- Gregory Ball
- 8 years ago
- Views:
Transcription
1 Guidance for Industry Food-Effect Bioavailability and Fed Bioequivalence Studies U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) December 2002 BP
2 Guidance for Industry Food-Effect Bioavailability and Fed Bioequivalence Studies Additional copies are available from: Office of Training and Communications Division of Drug Information, HFD Fishers Lane Rockville, MD (Tel) (Internet) U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) December 2002 BP
3 Table of Contents I. INTRODUCTION... 1 II. BACKGROUND...1 A. Potential Mechanisms of Food Effects on BA...1 B. Food Effects on Drug Products...2 III. RECOMMENDATIONS FOR FOOD-EFFECT BA AND FED BE STUDIES... 3 A. Immediate-Release Drug Products...3 B. Modified-Release Drug Products...4 IV. STUDY CONSIDERATIONS... 4 A. General Design...4 B. Subject Selection...5 C. Dosage Strength...5 D. Test Meal...5 E. Administration...6 F. Sample Collection...6 V. DATA ANALYSIS AND LABELING... 6 VI. OTHER CONSIDERATIONS... 8 A. Sprinkles...8 B. Special Vehicles...8
4 Guidance For Industry 1 Food-Effect Bioavailability and Fed Bioequivalence Studies This guidance represents the Food and Drug Administration=s current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. An alternative approach may be used if such approach satisfies the requirements of the applicable statutes and regulations. I. INTRODUCTION This guidance provides recommendations to sponsors and/or applicants planning to conduct food-effect bioavailability (BA) and fed bioequivalence (BE) studies for orally administered drug products as part of investigational new drug applications (INDs), new drug applications (NDAs), abbreviated new drug applications (ANDAs), and supplements to these applications. This guidance applies to both immediate-release and modified-release drug products. The guidance addresses how to meet the BA and BE requirements in 21 CFR 320, (d) (3), and (a) (7) as they apply to oral dosage forms. This guidance provides recommendations for food-effect BA and fed BE study designs, data analysis, and product labeling. It also provides information on when food-effect BA and fed BE studies should be performed. 2 II. BACKGROUND Food effect BA studies are usually conducted for new drugs and drug products during the IND period to assess the effects of food on the rate and extent of absorption of a drug when the drug product is administered shortly after a meal (fed conditions), as compared to administration under fasting conditions. Fed BE studies, on the other hand, are conducted for ANDAs to demonstrate their bioequivalence to the reference listed drug (RLD) under fed conditions. A. Potential Mechanisms of Food Effects on BA 1 This guidance has been prepared by the Food Effect Working Group of the Biopharmaceutics Coordinating Committee in the Office of Pharmaceutical Science, Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA). 2 See also the guidance for industry on Bioavailablity and Bioequivalence Studies for Orally Administered Drug Products C General Considerations. 1
5 Food can change the BA of a drug and can influence the BE between test and reference products. Food effects on BA can have clinically significant consequences. Food can alter BA by various means, including Delay gastric emptying Stimulate bile flow Change gastrointestinal (GI) ph Increase splanchnic blood flow Change luminal metabolism of a drug substance Physically or chemically interact with a dosage form or a drug substance Food effects on BA are generally greatest when the drug product is administered shortly after a meal is ingested. The nutrient and caloric contents of the meal, the meal volume, and the meal temperature can cause physiological changes in the GI tract in a way that affects drug product transit time, luminal dissolution, drug permeability, and systemic availability. In general, meals that are high in total calories and fat content are more likely to affect the GI physiology and thereby result in a larger effect on the BA of a drug substance or drug product. We recommend use of high-calorie and high-fat meals during food-effect BA and fed BE studies. B. Food Effects on Drug Products Administration of a drug product with food may change the BA by affecting either the drug substance or the drug product. In practice, it is difficult to determine the exact mechanism by which food changes the BA of a drug product without performing specific mechanistic studies. Important food effects on BA are least likely to occur with many rapidly dissolving, immediaterelease drug products containing highly soluble and highly permeable drug substances (BCS Class I) because absorption of the drug substances in Class I is usually ph- and site-independent and thus insensitive to differences in dissolution. 3 However, for some drugs in this class, food can influence BA when there is a high first-pass effect, extensive adsorption, complexation, or instability of the drug substance in the GI tract. In some cases, excipients or interactions between excipients and the food-induced changes in gut physiology can contribute to these food effects and influence the demonstration of BE. For rapidly dissolving formulations of BCS Class I drug substances, food can affect C max and the time at which this occurs (T max ) by delaying gastric emptying and prolonging intestinal transit time. However, we expect the food effect on these measures to be similar for test and reference products in fed BE studies. For other immediate-release drug products (BCS Class II, III, and IV) and for all modifiedrelease drug products, food effects are most likely to result from a more complex combination of factors that influence the in vivo dissolution of the drug product and/or the absorption of the drug substance. In these cases, the relative direction and magnitude of food effects on formulation BA and the effects on the demonstration of BE are difficult, if not impossible, to predict without conducting a fed BE study. 3 See the guidance for industry on Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. 2
6 III. RECOMMENDATIONS FOR FOOD-EFFECT BA AND FED BE STUDIES This section of the guidance provides recommendations on when food-effect BA studies should be conducted as part of INDs and NDAs and when fed BE studies should be conducted as part of ANDAs. For postapproval changes in an approved immediate- or modified-release drug product that requires in vivo redocumentation of BE under fasting conditions, fed BE studies are generally unnecessary. A. Immediate-Release Drug Products 1. INDs/NDAs We recommend that a food-effect BA study be conducted for all new chemical entities (NCEs) during the IND period. Food-effect BA studies should be conducted early in the drug development process to guide and select formulations for further development. Food-effect BA information should be available to design clinical safety and efficacy studies and to provide information for the CLINICAL PHARMACOLOGY and/or DOSAGE AND ADMINISTRATION sections of product labels. If a sponsor makes changes in components, composition, and/or method of manufacture in the clinical trial formulation prior to approval, BE should be demonstrated between the to-be-marketed formulation and the clinical trial formulation. Sponsors may wish to use relevant principles described in the guidance for industry on SUPAC-IR: Immediate Release Solid Oral Dosage Forms: Scale-Up and Post-Approval Changes: Chemistry, Manufacturing, and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation (SUPAC-IR guidance) to determine if in vivo BE studies are recommended. These BE studies, if indicated, should generally be conducted under fasting conditions. 2. ANDAs In addition to a BE study under fasting conditions, we recommend a BE study under fed conditions for all orally administered immediate-release drug products, with the following exceptions: When both test product and RLD are rapidly dissolving, have similar dissolution profiles, and contain a drug substance with high solubility and high permeability (BCS Class I) (see footnote 3), or When the DOSAGE AND ADMINISTRATION section of the RLD label states that the product should be taken only on an empty stomach, or 3
7 When the RLD label does not make any statements about the effect of food on absorption or administration. B. Modified-Release Drug Products We recommend that food-effect BA and fed BE studies be performed for all modifiedrelease dosage forms. 1. INDs/NDAs We recommend a study comparing the BA under fasting and fed conditions for all orally administered modified-release drug products. When changes occur in components, composition, and/or method of manufacture between the to-be-marketed formulation and the primary clinical trial material, the sponsor may wish to use relevant principles described in the guidance for industry on SUPAC-MR: Modified Release Solid Oral Dosage Forms: Scale-Up and Post-Approval Changes: Chemistry, Manufacturing, and Controls: In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation (SUPAC-MR guidance) to determine if documentation of in vivo BE is recommended. These BE studies, if indicated, should generally be conducted under fasting conditions. 2. ANDAs In addition to a BE study under fasting conditions, a BE study under fed conditions should be conducted for all orally administered modified-release drug products. IV. STUDY CONSIDERATIONS This section provides general considerations for designing food effect BA and fed BE studies. A sponsor may propose alternative study designs and data analyses. The scientific rationale and justification for these study designs and analyses should be provided in the study protocol. Sponsors may choose to conduct additional studies for a better understanding of the drug product and to provide optimal labeling statements for dosage and administration (e.g. different meals and different times of drug intake in relation to meals). In studying modified-release dosage forms, consideration should be given to the possibility that co-administration with food can result in dose dumping, in which the complete dose may be more rapidly released from the dosage form than intended, creating a potential safety risk for the study subjects. A. General Design We recommend a randomized, balanced, single-dose, two-treatment (fed vs. fasting), two-period, two-sequence crossover design for studying the effects of food on the BA of either an immediate-release or a modified-release drug product. The formulation to be tested should be administered on an empty stomach (fasting condition) in one period and following a test meal 4
8 (fed condition) in the other period. We recommend a similar, two-treatment, two-period, twosequence crossover design for a fed BE study except that the treatments should consist of both test and reference formulations administered following a test meal (fed condition). An adequate washout period should separate the two treatments in food-effect BA and fed BE studies. B. Subject Selection Both food-effect BA and fed BE studies can be carried out in healthy volunteers drawn from the general population. Studies in the patient population are also appropriate if safety concerns preclude the enrollment of healthy subjects. A sufficient number of subjects should complete the study to achieve adequate power for a statistical assessment of food effects on BA to claim an absence of food effects, or to claim BE in a fed BE study (see DATA ANALYSIS AND LABELING section). A minimum of 12 subjects should complete the food-effect BA and fed BE studies. C. Dosage Strength In general, the highest strength of a drug product intended to be marketed should be tested in food-effect BA and fed BE studies. In some cases, clinical safety concerns can prevent the use of the highest strength and warrant the use of lower strengths of the dosage form. For ANDAs, the same lot and strength used in the fasting BE study should be tested in the fed BE study. For products with multiple strengths in ANDAs, if a fed BE study has been performed on the highest strength, BE determination of one or more lower strengths can be waived based on dissolution profile comparisons (for details see the guidance on Bioavailablity and Bioequivalence Studies for Orally Administered Drug Products - General Considerations. D. Test Meal We recommend that food-effect BA and fed BE studies be conducted using meal conditions that are expected to provide the greatest effects on GI physiology so that systemic drug availability is maximally affected. A high-fat (approximately 50 percent of total caloric content of the meal) and high-calorie (approximately 800 to 1000 calories) meal is recommended as a test meal for food-effect BA and fed BE studies. This test meal should derive approximately 150, 250, and calories from protein, carbohydrate, and fat, respectively. 4 The caloric breakdown of the test meal should be provided in the study report. If the caloric breakdown of the meal is significantly different from the one described above, the sponsor should provide a scientific rationale for this difference. In NDAs, it is recognized that a sponsor can choose to conduct food-effect BA studies using meals with different combinations of fats, carbohydrates, and proteins for exploratory or label purposes. However, one of the meals for the food-effect BA studies should be the high-fat, high-calorie test meal described above. 4 An example test meal would be two eggs fried in butter, two strips of bacon, two slices of toast with butter, four ounces of hash brown potatoes and eight ounces of whole milk. Substitutions in this test meal can be made as long as the meal provides a similar amount of calories from protein, carbohydrate, and fat and has comparable meal volume and viscosity. 5
9 E. Administration Fasted Treatments: Following an overnight fast of at least 10 hours, subjects should be administered the drug product with 240 ml (8 fluid ounces) of water. No food should be allowed for at least 4 hours post-dose. Water can be allowed as desired except for one hour before and after drug administration. Subjects should receive standardized meals scheduled at the same time in each period of the study. Fed Treatments: Following an overnight fast of at least 10 hours, subjects should start the recommended meal 30 minutes prior to administration of the drug product. Study subjects should eat this meal in 30 minutes or less; however, the drug product should be administered 30 minutes after start of the meal. The drug product should be administered with 240 ml (8 fluid ounces) of water. No food should be allowed for at least 4 hours post-dose. Water can be allowed as desired except for one hour before and after drug administration. Subjects should receive standardized meals scheduled at the same time in each period of the study. F. Sample Collection For both fasted and fed treatment periods, timed samples in biological fluid, usually plasma, should be collected from the subjects to permit characterization of the complete shape of the plasma concentration-time profile for the parent drug. It may be advisable to measure other moieties in the plasma, such as active metabolites, and sponsors should refer to the guidance on Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations for recommendations on these issues. Consideration should be given to the possibility that co-administration of a dosage form with food can alter the time course of plasma drug concentrations so that fasted and fed treatments can have different sample collection times. V. DATA ANALYSIS AND LABELING Food-effect BA studies may be exploratory and descriptive, or a sponsor may want to use a foodeffect BA study to make a label claim. 5 The following exposure measures and pharmacokinetic parameters should be obtained from the resulting concentration-time curves for the test and reference products in food-effect BA and fed BE studies: Total exposure, or area under the concentration-time curve (AUC 0-inf, AUC 0-t ) Peak exposure (C max ) Time to peak exposure (T max ) Lag-time (t lag ) for modified-release products, if present Terminal elimination half-life Other relevant pharmacokinetic parameters Individual subject measurements, as well as summary statistics (e.g., group averages, standard deviations, coefficients of variation) should be reported. An equivalence approach is 5 Regulations on labeling requirements for a drug product submitted in an NDA can be found in 21 CFR part
10 recommended for food-effect BA (to make a claim of no food effects) and fed BE studies, analyzing data using an average criterion. Log-transformation of exposure measurements (AUC and C max ) prior to analysis is recommended. The 90 percent CI for the ratio of population geometric means between test and reference products should be provided for AUC 0-inf, AUC 0-t, and C max (see guidance for industry on Statistical Approaches to Establishing Bioequivalence). For IND or NDA food-effect BA studies, the fasted treatment serves as the reference. For ANDA fed BE studies, the RLD administered under fed condition serves as the reference treatment. The effect of food on the absorption and BA of a drug product should be described in the CLINICAL PHARMACOLOGY section of the labeling. In addition, the DOSAGE AND ADMINISTRATION section of the labeling should provide instructions for drug administration in relation to food based on clinical relevance (i.e., whether or not the changes in systemic exposure caused by co-administration with food results in safety or efficacy concerns, or when there is no important change in systemic exposure but there is a possibility that the drug substance causes GI irritation when taken without food). For an NDA, an absence of food effect on BA is not established if the 90 percent CI for the ratio of population geometric means between fed and fasted treatments, based on log-transformed data, is not contained in the equivalence limits of percent for either AUC 0-inf (AUC 0-t when appropriate) or C max. When the 90 percent CI fails to meet the limits of percent, the sponsor should provide specific recommendations on the clinical significance of the food effect based on what is known from the total clinical database about dose-response (exposureresponse) and/or pharmacokinetic-pharmacodynamic relationships of the drug under study. The clinical relevance of any difference in T max and t lag should also be indicated by the sponsor. The results of the food-effect BA study should be reported factually in the CLINICAL PHARMACOLOGY section of the labeling and should form the basis for making label recommendations (e.g., take only on an empty stomach) in the DOSAGE AND ADMINISTRATION section of the labeling. The following are examples of language for the package insert: A food-effect study involving administration of [the drug product] to healthy volunteers under fasting conditions and with a high-fat meal indicated that the C max and AUC were increased 57% and 45%, respectively, under fed conditions. This increase in exposure can be clinically significant, and therefore [the drug] should be taken only on an empty stomach (1 hour before or 2 hours after a meal) A food-effect study involving administration of [the drug product] to healthy volunteers under fasting conditions and with a high-fat meal indicated that the C max was decreased 15% while the AUC remained unchanged. This decrease in exposure is not clinically significant, and therefore [the drug] could be taken without regards to meals. An absence of food effect on BA is indicated when the 90 percent CI for the ratio of population geometric means between fed and fasted treatments, based on log-transformed data, is contained in the equivalence limits of percent for AUC 0-inf (AUC 0-t when appropriate) and C max. In this case, a sponsor can make a specific claim in the CLINICAL PHARMACOLOGY or DOSAGE AND ADMINISTRATION section of the label that no food effect on BA is expected 7
11 provided that the T max differences between the fasted and fed treatments are not clinically relevant. The following is an example of language for the package insert: The C max and AUC data from a food-effect study involving administration of [the drug product] to healthy volunteers under fasting conditions and with a high-fat meal indicated that exposure to the drug is not affected by food. Therefore, [the drug product] may be taken without regard to meals. For an ANDA, BE of a test product to the RLD product under fed conditions is concluded when the 90 percent CI for the ratio of population geometric means between the test and RLD product, based on log-transformed data, is contained in the BE limits of percent for AUC and C max. Although no criterion applies to T max, the T max values for the test and reference products are expected to be comparable based on clinical relevance. The conclusion of BE under fed conditions indicates that with regard to food, the language in the package insert of the test product can be the same as the reference product. VI. OTHER CONSIDERATIONS A. Sprinkles In NDAs, the labeling of certain drug products (e.g., controlled-release capsules containing beads) can recommend that the product be sprinkled on soft foods, such as applesauce, and swallowed without chewing. For the labeling to indicate that the drug product can be sprinkled on soft foods, additional in vivo relative BA studies should be performed by sprinkling the product on the soft foods to be listed in the labeling (test treatment) and comparing it to the product administered in the intact form (reference treatment), then administering both on an empty stomach. In ANDAs, BE of the test to the RLD is demonstrated in a single dose crossover study. Both treatments should be sprinkled on one of the soft foods mentioned in the labeling, usually applesauce. The BE data should be analyzed using average BE and the 90 percent CI criteria should be used to declare BE. If there are questions about other foods, the design, or the analysis of such BE studies, the sponsors and/or applicants should contact the Office of Generic Drugs. B. Special Vehicles For NDAs, the labeling for certain oral solution products (e.g., cyclosporine oral solution, modified) recommends that the solution be mixed with a beverage prior to administration. The BA of these products can change when mixed with different beverages due to the formation of complex mixtures and other physical-chemical and/or physiological factors. NDA sponsors should contact the Office of Clinical Pharmacology and Biopharmaceutics to determine what data should be submitted to support labeling. In ANDAs, BE of the test to the RLD is demonstrated in a single-dose crossover study. Both treatments should be mixed with one of the beverages mentioned in the labeling. Sponsors 8
12 should provide evidence that BE differences would not be expected from the use of other listed vehicles. The BE data should be analyzed using average BE, and the 90 percent CI criteria should be used to declare BE. If there are questions about other vehicles, or the design or analysis of such BE studies, the sponsors and/or applicants should contact the Office of Generic Drugs. 9
Guidance for Industry
 Guidance for Industry Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry
 Guidance for Industry Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs General Considerations DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Guidance for Industry Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs General Considerations DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Guidance for Industry
 Guidance for Industry Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Guidance for Industry Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Overview of Dissolution for BA/BE
 Biopharmaceutics Classification System based on Solubility/Permeability Biowaivers for BCS I Drugs Discussion of BCS III Drugs Models establishing in vivo-in vitro Correlations (IVIVC Levels A-C) 1 Biopharmaceutics
Biopharmaceutics Classification System based on Solubility/Permeability Biowaivers for BCS I Drugs Discussion of BCS III Drugs Models establishing in vivo-in vitro Correlations (IVIVC Levels A-C) 1 Biopharmaceutics
Bioequivalence Study Design Considerations. Dr. John Gordon
 Bioequivalence Study Design Considerations Dr. John Gordon Key Output of Programme A list of prequalified medicinal products used for treatment of HIV/AIDS, malaria, tuberculosis, influenza, and for reproductive
Bioequivalence Study Design Considerations Dr. John Gordon Key Output of Programme A list of prequalified medicinal products used for treatment of HIV/AIDS, malaria, tuberculosis, influenza, and for reproductive
Guidance for Industry
 #171 Guidance for Industry Waivers of In Vivo Demonstration of Bioequivalence of Animal Drugs in Soluble Powder Oral Dosage Form Products and Type A Medicated Articles (This version of the guidance replaces
#171 Guidance for Industry Waivers of In Vivo Demonstration of Bioequivalence of Animal Drugs in Soluble Powder Oral Dosage Form Products and Type A Medicated Articles (This version of the guidance replaces
Guidance for Industry
 Guidance for Industry Immediate Release Solid Oral Dosage Forms Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation
Guidance for Industry Immediate Release Solid Oral Dosage Forms Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation
Guidance for Industry
 Guidance for Industry Major, Minor, and Telephone Amendments to Abbreviated New Drug Applications Comments and suggestions regarding this document should be submitted within 90 days of publication in the
Guidance for Industry Major, Minor, and Telephone Amendments to Abbreviated New Drug Applications Comments and suggestions regarding this document should be submitted within 90 days of publication in the
MULTISOURCE (GENERIC) PHARMACEUTICAL PRODUCTS: GUIDELINES ON REGISTRATION REQUIREMENTS TO ESTABLISH INTERCHANGEABILITY. REVISION (JULY 2014)
 1 Working document QAS/14.583/Rev.1 July 2014 Document for comment 2 3 4 5 6 7 8 9 MULTISOURCE (GENERIC) PHARMACEUTICAL PRODUCTS: GUIDELINES ON REGISTRATION REQUIREMENTS TO ESTABLISH INTERCHANGEABILITY.
1 Working document QAS/14.583/Rev.1 July 2014 Document for comment 2 3 4 5 6 7 8 9 MULTISOURCE (GENERIC) PHARMACEUTICAL PRODUCTS: GUIDELINES ON REGISTRATION REQUIREMENTS TO ESTABLISH INTERCHANGEABILITY.
Absorption of Drugs. Transport of a drug from the GI tract
 Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System Guidance for Industry DRAFT GUIDANCE This guidance
Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System Guidance for Industry DRAFT GUIDANCE This guidance
Guidance for Industry
 Guidance for Industry Dissolution Testing of Immediate Release Solid Oral Dosage Forms U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research
Guidance for Industry Dissolution Testing of Immediate Release Solid Oral Dosage Forms U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research
Post-Approval Change Management: Challenges and Opportunities An FDA Perspective
 CMC Workshop From Drug Development to Global Supply to Patients April 15-17, 2013, Washington, DC Post-Approval Change Management: Challenges and Opportunities An FDA Perspective Christine M. V. Moore,
CMC Workshop From Drug Development to Global Supply to Patients April 15-17, 2013, Washington, DC Post-Approval Change Management: Challenges and Opportunities An FDA Perspective Christine M. V. Moore,
MULTISOURCE (GENERIC) PHARMACEUTICAL PRODUCTS: GUIDELINES ON REGISTRATION REQUIREMENTS TO ESTABLISH INTERCHANGEABILITY DRAFT REVISION
 RESTRICTED WORLD HEALTH ORGANIZATION ORGANISATION MONDIALE DE LA SANTE MULTISOURCE (GENERIC) PHARMACEUTICAL PRODUCTS: GUIDELINES ON REGISTRATION REQUIREMENTS TO ESTABLISH INTERCHANGEABILITY DRAFT REVISION
RESTRICTED WORLD HEALTH ORGANIZATION ORGANISATION MONDIALE DE LA SANTE MULTISOURCE (GENERIC) PHARMACEUTICAL PRODUCTS: GUIDELINES ON REGISTRATION REQUIREMENTS TO ESTABLISH INTERCHANGEABILITY DRAFT REVISION
Guidance for Industry
 Guidance for Industry Fixed Dose Combinations, Co-Packaged Drug Products, and Single-EntityVersions of Previously Approved Antiretrovirals for the Treatment of HIV U.S. Department of Health and Human Services
Guidance for Industry Fixed Dose Combinations, Co-Packaged Drug Products, and Single-EntityVersions of Previously Approved Antiretrovirals for the Treatment of HIV U.S. Department of Health and Human Services
Bundesinstitut für Arzneimittel und Medizinprodukte. Dissolution Testing. Analytik,Methodenentwicklung, Bioäquivalenz SAQ. Olten, 25.
 Dissolution Testing Analytik,Methodenentwicklung, Bioäquivalenz SAQ Olten, 25. Januar 2006 Dr. H. Potthast (h.potthast@bfarm.de) 1 2 Basis for Biowaiver Applications/Decisions Note for Guidance on the
Dissolution Testing Analytik,Methodenentwicklung, Bioäquivalenz SAQ Olten, 25. Januar 2006 Dr. H. Potthast (h.potthast@bfarm.de) 1 2 Basis for Biowaiver Applications/Decisions Note for Guidance on the
Compilation of individual product-specific guidance on demonstration of bioequivalence
 17 December 2014 EMA/CHMP/736403/2014 Committee for Medicinal Products for Human Use (CHMP) Compilation of individual product-specific guidance on demonstration of bioequivalence Initial batch of individual
17 December 2014 EMA/CHMP/736403/2014 Committee for Medicinal Products for Human Use (CHMP) Compilation of individual product-specific guidance on demonstration of bioequivalence Initial batch of individual
Guidance for Industry
 Guidance for Industry Applications Covered by Section 505(b)(2) DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
Guidance for Industry Applications Covered by Section 505(b)(2) DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
February 2006 Procedural
 Guidance for Industry Reports on the Status of Postmarketing Study Commitments Implementation of Section 130 of the Food and Drug Administration Modernization Act of 1997 U.S. Department of Health and
Guidance for Industry Reports on the Status of Postmarketing Study Commitments Implementation of Section 130 of the Food and Drug Administration Modernization Act of 1997 U.S. Department of Health and
A Peak at PK An Introduction to Pharmacokinetics
 Paper IS05 A Peak at PK An Introduction to Pharmacokinetics Hannah Twitchett, Roche Products Ltd, Welwyn Garden City, UK Paul Grimsey, Roche Products Ltd, Welwyn Garden City, UK ABSTRACT The aim of this
Paper IS05 A Peak at PK An Introduction to Pharmacokinetics Hannah Twitchett, Roche Products Ltd, Welwyn Garden City, UK Paul Grimsey, Roche Products Ltd, Welwyn Garden City, UK ABSTRACT The aim of this
Guidance for Industry
 Guidance for Industry Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product DRAFT GUIDANCE This guidance document is being distributed for comment purposes only.
Guidance for Industry Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product DRAFT GUIDANCE This guidance document is being distributed for comment purposes only.
Guidance for Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation
 Guidance for Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Guidance for Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Letter Date March 27, 2007 Stamp Date March 28, 2007 PDUFA Goal Date January 28, 2008
 CLINICAL REVIEW Application Type NDA Submission Number 22-157 Submission Code Letter Date March 27, 2007 Stamp Date March 28, 2007 PDUFA Goal Date January 28, 2008 Reviewer Name Review Completion Date
CLINICAL REVIEW Application Type NDA Submission Number 22-157 Submission Code Letter Date March 27, 2007 Stamp Date March 28, 2007 PDUFA Goal Date January 28, 2008 Reviewer Name Review Completion Date
Generic drugs are copies of innovator drug products
 dx.doi.org/10.14227/dt190412p51 In Vitro Equivalence Studies of Generic Metformin Hydrochloride Tablets and Propranolol Hydrochloride Tablets Under Biowaiver Conditions in Lagos State, Nigeria e-mail:
dx.doi.org/10.14227/dt190412p51 In Vitro Equivalence Studies of Generic Metformin Hydrochloride Tablets and Propranolol Hydrochloride Tablets Under Biowaiver Conditions in Lagos State, Nigeria e-mail:
Guidance for Industry
 Guidance for Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry
 Guidance for Industry Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Guidance for Industry Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
Guidance for Industry
 Guidance for Industry Q2B Validation of Analytical Procedures: Methodology November 1996 ICH Guidance for Industry Q2B Validation of Analytical Procedures: Methodology Additional copies are available from:
Guidance for Industry Q2B Validation of Analytical Procedures: Methodology November 1996 ICH Guidance for Industry Q2B Validation of Analytical Procedures: Methodology Additional copies are available from:
BIOAVAILABILITY & BIOEQUIVALENCE TRIALS
 BIOAVAILABILITY & BIOEQUIVALENCE TRIALS Shubha Rani,, Ph.D. Technical Director & Head-Biometrics and Data Management Synchron Research Services Pvt. Ltd. Ahmedabad 380 054 drshubha@synchronresearch.com
BIOAVAILABILITY & BIOEQUIVALENCE TRIALS Shubha Rani,, Ph.D. Technical Director & Head-Biometrics and Data Management Synchron Research Services Pvt. Ltd. Ahmedabad 380 054 drshubha@synchronresearch.com
Guideline on the conduct of bioequivalence studies for veterinary medicinal products
 11 April 2011 EMA/CVMP/016/00-Rev.2 Committee for Medicinal Products for Veterinary Use (CVMP) Guideline on the conduct of bioequivalence studies for veterinary medicinal products Draft revised GL agreed
11 April 2011 EMA/CVMP/016/00-Rev.2 Committee for Medicinal Products for Veterinary Use (CVMP) Guideline on the conduct of bioequivalence studies for veterinary medicinal products Draft revised GL agreed
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 29 July 1999 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON QUALITY OF MODIFIED
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 29 July 1999 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON QUALITY OF MODIFIED
Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules
 Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry
 Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry
 Guidance for Industry IND Exemptions for Studies of Lawfully Marketed Drug or Biological Products for the Treatment of Cancer U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry IND Exemptions for Studies of Lawfully Marketed Drug or Biological Products for the Treatment of Cancer U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry Determining the Extent of Safety Data Collection Needed in Late Stage Premarket and Postapproval Clinical Investigations
 Guidance for Industry Determining the Extent of Safety Data Collection Needed in Late Stage Premarket and Postapproval Clinical Investigations DRAFT GUIDANCE This guidance document is being distributed
Guidance for Industry Determining the Extent of Safety Data Collection Needed in Late Stage Premarket and Postapproval Clinical Investigations DRAFT GUIDANCE This guidance document is being distributed
Public Assessment Report Scientific discussion. Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC
 Public Assessment Report Scientific discussion Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC This module reflects the scientific discussion for the approval of Tenofovir disoproxil Teva.
Public Assessment Report Scientific discussion Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC This module reflects the scientific discussion for the approval of Tenofovir disoproxil Teva.
ANDA CHECKLIST FOR CTD or ectd FORMAT FOR COMPLETENESS and ACCEPTABILITY of an APPLICATION FOR FILING
 ANDA CHECKLIST FOR CTD or ectd FORMAT FOR COMPLETENESS and ACCEPTABILITY of an APPLICATION FOR FILING For More Information on Submission of an ANDA in Electronic Common Technical Document (ectd) Format
ANDA CHECKLIST FOR CTD or ectd FORMAT FOR COMPLETENESS and ACCEPTABILITY of an APPLICATION FOR FILING For More Information on Submission of an ANDA in Electronic Common Technical Document (ectd) Format
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA
 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA VIROPHARMA INCORPORATED, 730 Stockton Drive, Exton, PA 19341, Plaintiff, Civil Action No. v. MARGARET A. HAMBURG, M.D., in her official
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF COLUMBIA VIROPHARMA INCORPORATED, 730 Stockton Drive, Exton, PA 19341, Plaintiff, Civil Action No. v. MARGARET A. HAMBURG, M.D., in her official
Liposome Drug Products
 Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation Guidance for Industry DRAFT GUIDANCE This guidance document is being
Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation Guidance for Industry DRAFT GUIDANCE This guidance document is being
Food, Medicine and Health Care Administration and Control Authority
 Food, Medicine and Health Care Administration and Control Authority Bio equivalence Study Registration Requirements in Ethiopia (Four Countries Experience) Mengistab W.Aregay (Bpharm, MSc. in Health Monitoring
Food, Medicine and Health Care Administration and Control Authority Bio equivalence Study Registration Requirements in Ethiopia (Four Countries Experience) Mengistab W.Aregay (Bpharm, MSc. in Health Monitoring
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Guidance for Industry Safety Testing of Drug Metabolites
 Guidance for Industry Safety Testing of Drug Metabolites U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2008 Pharmacology
Guidance for Industry Safety Testing of Drug Metabolites U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2008 Pharmacology
EXIGENCIA DE ESTUDIOS DE BIOEQUIVALENCIA A TRAVÉS DE METODOS IN VITRO
 EXIGENCIA DE ESTUDIOS DE BIOEQUIVALENCIA A TRAVÉS DE METODOS IN VITRO Q.F. ALEXIS ACEITUNO, PhD Jefe Subdepto. Biofarmacia & Bioequivalencia Agencia Nacional de Medicamentos Instituto de Salud Pública
EXIGENCIA DE ESTUDIOS DE BIOEQUIVALENCIA A TRAVÉS DE METODOS IN VITRO Q.F. ALEXIS ACEITUNO, PhD Jefe Subdepto. Biofarmacia & Bioequivalencia Agencia Nacional de Medicamentos Instituto de Salud Pública
Guidance for Industry
 #238 Guidance for Industry Modified Release Veterinary Parenteral Dosage Forms: Development, Evaluation, and Establishment of Specifications DRAFT GUIDANCE This guidance document is being distributed for
#238 Guidance for Industry Modified Release Veterinary Parenteral Dosage Forms: Development, Evaluation, and Establishment of Specifications DRAFT GUIDANCE This guidance document is being distributed for
Guidance for Industry and Investigators Safety Reporting Requirements for INDs and BA/BE Studies- Small Entity Compliance Guide
 Guidance for Industry and Investigators Safety Reporting Requirements for INDs and BA/BE Studies- Small Entity Compliance Guide U.S. Department of Health and Human Services Center for Drug Evaluation and
Guidance for Industry and Investigators Safety Reporting Requirements for INDs and BA/BE Studies- Small Entity Compliance Guide U.S. Department of Health and Human Services Center for Drug Evaluation and
HYDROCORTISONE 10 MG TABLETS
 HYDROCORTISONE 10 MG TABLETS (Hydrocortisone) PL 20072/0238 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation summary
HYDROCORTISONE 10 MG TABLETS (Hydrocortisone) PL 20072/0238 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation summary
CTD Dossier Preparation. Sr.Manager-Regulatory Affairs
 CTD Dossier Preparation K. Srikantha Reddy Sr.Manager-Regulatory Affairs Medreich Limited Srikanth.k@medreich.com CTD Dossier Preparation CTD (Common Technical Document) contains 5 modules Module 1 Module
CTD Dossier Preparation K. Srikantha Reddy Sr.Manager-Regulatory Affairs Medreich Limited Srikanth.k@medreich.com CTD Dossier Preparation CTD (Common Technical Document) contains 5 modules Module 1 Module
Guidance for Industry
 Guidance for Industry Changes to an Approved NDA or ANDA U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) April 2004 CMC Revision
Guidance for Industry Changes to an Approved NDA or ANDA U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) April 2004 CMC Revision
Bioequivalence Testing, using the Dissolution Profile
 Determining Similarity of Products- F 2 Criterion and Variability of Dissolution Test Vivian Gray V. A. Gray Consulting Dissolution Workshop December 10, 2010 Bioequivalence Testing, using the Dissolution
Determining Similarity of Products- F 2 Criterion and Variability of Dissolution Test Vivian Gray V. A. Gray Consulting Dissolution Workshop December 10, 2010 Bioequivalence Testing, using the Dissolution
Sweet-taste receptors, glucose absorption and insulin release: Are LCS nutritionally active?
 Sweet-taste receptors, glucose absorption and insulin release: Are LCS nutritionally active? Samuel V. Molinary, Ph.D. Consultant, Scientific & Regulatory Affairs ILSI/NA April 6, 2011 Washington, DC Why
Sweet-taste receptors, glucose absorption and insulin release: Are LCS nutritionally active? Samuel V. Molinary, Ph.D. Consultant, Scientific & Regulatory Affairs ILSI/NA April 6, 2011 Washington, DC Why
Public Assessment Report. Scientific discussion. Desloracell 5 mg, film-coated tablet. (desloratadine) NL License RVG: 112807
 Public Assessment Report Scientific discussion Desloracell 5 mg, film-coated tablet (desloratadine) NL License RVG: 112807 Date: 6 July 2015 This module reflects the scientific discussion for the approval
Public Assessment Report Scientific discussion Desloracell 5 mg, film-coated tablet (desloratadine) NL License RVG: 112807 Date: 6 July 2015 This module reflects the scientific discussion for the approval
Humulin (LY041001) Page 1 of 1
 (LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
(LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
Guidance for Industry
 Guidance for Industry Exposure-Response Relationships Study Design, Data Analysis, and Regulatory Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug
Guidance for Industry Exposure-Response Relationships Study Design, Data Analysis, and Regulatory Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Guidance for Industry ANDAs: Stability Testing of Drug Substances and Products
 Guidance for Industry ANDAs: Stability Testing of Drug Substances and Products Questions and Answers U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry ANDAs: Stability Testing of Drug Substances and Products Questions and Answers U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry
 Guidance for Industry Scientific Considerations in Demonstrating Biosimilarity to a Reference Product DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and
Guidance for Industry Scientific Considerations in Demonstrating Biosimilarity to a Reference Product DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and
GDUFA Regulatory Science Update
 GDUFA Regulatory Science Update Robert Lionberger, Ph.D. Director Office of Research and Standards Office of Generic Drugs Center for Drug Evaluation and Research, FDA GPhA Annual Meeting Feb 9, 2015 Goals
GDUFA Regulatory Science Update Robert Lionberger, Ph.D. Director Office of Research and Standards Office of Generic Drugs Center for Drug Evaluation and Research, FDA GPhA Annual Meeting Feb 9, 2015 Goals
Quality by Design for ANDAs: An Example for Modified Release Dosage Forms
 Quality by Design for ANDAs: An Example for Modified Release Dosage Forms Introduction to the Example This is an example pharmaceutical development report illustrating how ANDA applicants can move toward
Quality by Design for ANDAs: An Example for Modified Release Dosage Forms Introduction to the Example This is an example pharmaceutical development report illustrating how ANDA applicants can move toward
Clinical Pharmacology 1: Phase 1 Studies and Early Drug Development. Gerlie Gieser, Ph.D. Office of Clinical Pharmacology, Div. IV
 Clinical Pharmacology 1: Phase 1 Studies and Early Drug Development Gerlie Gieser, Ph.D. Office of Clinical Pharmacology, Div. IV Objectives Outline the Phase 1 studies conducted to characterize the Clinical
Clinical Pharmacology 1: Phase 1 Studies and Early Drug Development Gerlie Gieser, Ph.D. Office of Clinical Pharmacology, Div. IV Objectives Outline the Phase 1 studies conducted to characterize the Clinical
Generic Drug User Fee Act Program Performance Goals and Procedures
 Generic Drug User Fee Act Program Performance Goals and Procedures The performance efficiencies, metric goals and procedures to which FDA will agree upon commencement of a generic drug user fee act (GDUFA)
Generic Drug User Fee Act Program Performance Goals and Procedures The performance efficiencies, metric goals and procedures to which FDA will agree upon commencement of a generic drug user fee act (GDUFA)
Overview of Pre-Approval Inspections
 Overview of Pre-Approval Inspections Presented by: Kelli F. Dobilas NWJ-DO Pre-Approval Manager Pre-Approval Drug Inspections What are Pre-Approval Inspections? One of the last reviews of the drug approval
Overview of Pre-Approval Inspections Presented by: Kelli F. Dobilas NWJ-DO Pre-Approval Manager Pre-Approval Drug Inspections What are Pre-Approval Inspections? One of the last reviews of the drug approval
Oral Bioavailability of Creatine Supplements: Is There Room for Improvement?
 Oral Bioavailability of Creatine Supplements: Is There Room for Improvement? Donald W. Miller Associate Professor Department of Pharmacology and Therapeutics University of Manitoba Acknowledgements ISSN
Oral Bioavailability of Creatine Supplements: Is There Room for Improvement? Donald W. Miller Associate Professor Department of Pharmacology and Therapeutics University of Manitoba Acknowledgements ISSN
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
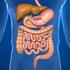 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
5.5 Pharmacokinetics - Sublingual
 5.5 Pharmacokinetics - Sublingual 5.5.1 Single Dose Pharmacokinetics - Dose Linearity Two studies in young healthy males were dedicated to examining sublingual single rising dose (SRD) pharmacokinetics
5.5 Pharmacokinetics - Sublingual 5.5.1 Single Dose Pharmacokinetics - Dose Linearity Two studies in young healthy males were dedicated to examining sublingual single rising dose (SRD) pharmacokinetics
Introduction to Enteris BioPharma
 Introduction to Enteris BioPharma Enteris BioPharma Intelligent Solutions for Oral Drug Delivery Privately held, New Jersey based biotech company Owned solely by Victory Park Capital, a large Chicago based
Introduction to Enteris BioPharma Enteris BioPharma Intelligent Solutions for Oral Drug Delivery Privately held, New Jersey based biotech company Owned solely by Victory Park Capital, a large Chicago based
Public Assessment Report. Decentralised Procedure
 Public Assessment Report Decentralised Procedure TELMISARTAN DR REDDY S 20 MG TABLETS TELMISARTAN DR REDDY S 40 MG TABLETS TELMISARTAN DR REDDY S 80 MG TABLETS (telmisartan) Procedure No: UK/H/5034/001-003/DC
Public Assessment Report Decentralised Procedure TELMISARTAN DR REDDY S 20 MG TABLETS TELMISARTAN DR REDDY S 40 MG TABLETS TELMISARTAN DR REDDY S 80 MG TABLETS (telmisartan) Procedure No: UK/H/5034/001-003/DC
Ibuprofen 200mg Soft Capsules PL 03105/0105 UKPAR
 Ibuprofen 200mg Soft Capsules PL 03105/0105 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 4 Steps taken for assessment Page 13 Summary of Product Characteristics Page 14 Patient
Ibuprofen 200mg Soft Capsules PL 03105/0105 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 4 Steps taken for assessment Page 13 Summary of Product Characteristics Page 14 Patient
POLICY AND PROCEDURES OFFICE OF NEW DRUGS. Good Review Practice: Management of Breakthrough Therapy-Designated Drugs and Biologics.
 POLICY AND PROCEDURES OFFICE OF NEW DRUGS Good Review Practice: Management of Breakthrough Therapy-Designated Drugs and Biologics Table of Contents PURPOSE...1 BACKGROUND...2 POLICY...3 ROLES AND RESPONSIBILITIES...4
POLICY AND PROCEDURES OFFICE OF NEW DRUGS Good Review Practice: Management of Breakthrough Therapy-Designated Drugs and Biologics Table of Contents PURPOSE...1 BACKGROUND...2 POLICY...3 ROLES AND RESPONSIBILITIES...4
The 505(b)(2) Drug Development Pathway:
 The 505(b)(2) Drug Development Pathway: When and How to Take Advantage of a Unique American Regulatory Pathway By Mukesh Kumar, PhD, RAC and Hemant Jethwani, MS The 505(b)(2) regulation offers a less expensive
The 505(b)(2) Drug Development Pathway: When and How to Take Advantage of a Unique American Regulatory Pathway By Mukesh Kumar, PhD, RAC and Hemant Jethwani, MS The 505(b)(2) regulation offers a less expensive
Guidance for Industry Formal Meetings Between the FDA and Sponsors or Applicants
 Guidance for Industry Formal Meetings Between the FDA and Sponsors or Applicants U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry Formal Meetings Between the FDA and Sponsors or Applicants U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry and FDA Staff FDA Acceptance of Foreign Clinical Studies Not Conducted Under an IND Frequently Asked Questions
 Guidance for Industry and FDA Staff FDA Acceptance of Foreign Clinical Studies Not Conducted Under an IND Frequently Asked Questions U.S. Department of Health and Human Services Food and Drug Administration
Guidance for Industry and FDA Staff FDA Acceptance of Foreign Clinical Studies Not Conducted Under an IND Frequently Asked Questions U.S. Department of Health and Human Services Food and Drug Administration
Introduction to pharmaceutical technology
 Introduction to pharmaceutical technology Marie Wahlgren Chapter 1 What is the topics of today Introduction to the course Introduction to the project assignment How to choose a new drug formulation 1 Contacts
Introduction to pharmaceutical technology Marie Wahlgren Chapter 1 What is the topics of today Introduction to the course Introduction to the project assignment How to choose a new drug formulation 1 Contacts
Guidance for Industry
 Guidance for Industry Providing Regulatory Submissions in Electronic Format Content of Labeling U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Providing Regulatory Submissions in Electronic Format Content of Labeling U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
CATEGORY Advertising. CATEGORY Biopharmaceutics. CATEGORY Biosimilarity
 CATEGORY Advertising Guidance Agenda: New & Guidances CDER is Planning to Publish During Calendar Year 2016 (See the Good Guidance Practices (GGPs) regulation on this Web page or 21 CFR 10.115 for details
CATEGORY Advertising Guidance Agenda: New & Guidances CDER is Planning to Publish During Calendar Year 2016 (See the Good Guidance Practices (GGPs) regulation on this Web page or 21 CFR 10.115 for details
Guidance for Industry
 Guidance for Industry Codevelopment of Two or More New Investigational Drugs for Use in Combination U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry Codevelopment of Two or More New Investigational Drugs for Use in Combination U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guideline for Industry
 Guideline for Industry The Extent of Population Exposure to Assess Clinical Safety: For Drugs Intended for Longterm Treatment of Non-Life- Threatening Conditions ICH-E1A March 1995 GUIDELINE FOR INDUSTRY
Guideline for Industry The Extent of Population Exposure to Assess Clinical Safety: For Drugs Intended for Longterm Treatment of Non-Life- Threatening Conditions ICH-E1A March 1995 GUIDELINE FOR INDUSTRY
Guidance for Industry Time and Extent Applications for Nonprescription Drug Products
 Guidance for Industry Time and Extent Applications for Nonprescription Drug Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
Guidance for Industry Time and Extent Applications for Nonprescription Drug Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
For personal use only
 6 July 2015 KEY ADVANCEMENT TOWARDS COMMENCEMENT OF PHASE 1 CLINICAL STUDY OF ORAL FORMULATIONS Highlights PhytoTech Medical has submitted key documents to the Institutional Review Board (IRB or Helsinki
6 July 2015 KEY ADVANCEMENT TOWARDS COMMENCEMENT OF PHASE 1 CLINICAL STUDY OF ORAL FORMULATIONS Highlights PhytoTech Medical has submitted key documents to the Institutional Review Board (IRB or Helsinki
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE INVESTIGATION OF BIOAVAILABILITY AND BIOEQUIVALENCE
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 14 December 2000 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 14 December 2000 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE
Not All Clinical Trials Are Created Equal Understanding the Different Phases
 Not All Clinical Trials Are Created Equal Understanding the Different Phases This chapter will help you understand the differences between the various clinical trial phases and how these differences impact
Not All Clinical Trials Are Created Equal Understanding the Different Phases This chapter will help you understand the differences between the various clinical trial phases and how these differences impact
Revision of The Dissolution Procedure: Development and Validation 1092
 Page 1 of 5 STIMULI TO THE REVISION PROCESS Stimuli articles do not necessarily reflect the policies of the USPC or the USP Council of Experts Revision of The Dissolution Procedure: Development and Validation
Page 1 of 5 STIMULI TO THE REVISION PROCESS Stimuli articles do not necessarily reflect the policies of the USPC or the USP Council of Experts Revision of The Dissolution Procedure: Development and Validation
Guidance for Industry
 Guidance for Industry Content and Format of Investigational New Drug Applications (INDs) for Phase 1 Studies of Drugs, Including Well-Characterized, Therapeutic, Biotechnology-derived Products Center for
Guidance for Industry Content and Format of Investigational New Drug Applications (INDs) for Phase 1 Studies of Drugs, Including Well-Characterized, Therapeutic, Biotechnology-derived Products Center for
Public Assessment Report UKPAR
 Public Assessment Report UKPAR Amitriptyline 10 mg film-coated tablets Amitriptyline 25 mg film-coated tablets Amitriptyline 50 mg film-coated tablets (Amitriptyline hydrochloride) UK Licence No: PL 17907/0131-133
Public Assessment Report UKPAR Amitriptyline 10 mg film-coated tablets Amitriptyline 25 mg film-coated tablets Amitriptyline 50 mg film-coated tablets (Amitriptyline hydrochloride) UK Licence No: PL 17907/0131-133
Statistics and Pharmacokinetics in Clinical Pharmacology Studies
 Paper ST03 Statistics and Pharmacokinetics in Clinical Pharmacology Studies ABSTRACT Amy Newlands, GlaxoSmithKline, Greenford UK The aim of this presentation is to show how we use statistics and pharmacokinetics
Paper ST03 Statistics and Pharmacokinetics in Clinical Pharmacology Studies ABSTRACT Amy Newlands, GlaxoSmithKline, Greenford UK The aim of this presentation is to show how we use statistics and pharmacokinetics
Adocia reports positive results from phase IIa clinical study of ultra-fast acting BioChaperone Lispro
 PRESS RELEASE Adocia reports positive results from phase IIa clinical study of ultra-fast acting BioChaperone Lispro BioChaperone Lispro is significantly faster than Humalog in type I diabetic patients;
PRESS RELEASE Adocia reports positive results from phase IIa clinical study of ultra-fast acting BioChaperone Lispro BioChaperone Lispro is significantly faster than Humalog in type I diabetic patients;
Guidance for Industry Independent Consultants for Biotechnology Clinical Trial Protocols
 Guidance for Industry Independent Consultants for Biotechnology Clinical Trial Protocols For questions on the content of this document contact Leonard Wilson, CBER at 301-827-0373 or Susan Johnson, CDER
Guidance for Industry Independent Consultants for Biotechnology Clinical Trial Protocols For questions on the content of this document contact Leonard Wilson, CBER at 301-827-0373 or Susan Johnson, CDER
M4E(R2): The CTD Efficacy
 M4E(R2): The CTD Efficacy This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA or Agency) on this topic. It does not establish any rights for
M4E(R2): The CTD Efficacy This draft guidance, when finalized, will represent the current thinking of the Food and Drug Administration (FDA or Agency) on this topic. It does not establish any rights for
Public Assessment Report. Decentralised Procedure. Omeprazole 10mg Gastro-resistant Capsules, hard. Omeprazole 20mg Gastro-resistant Capsules, hard
 Public Assessment Report Decentralised Procedure Omeprazole 10mg Gastro-resistant Capsules, hard Omeprazole 20mg Gastro-resistant Capsules, hard Omeprazole 40mg Gastro-resistant Capsules, hard (UK/H/4423/001-003/DC)
Public Assessment Report Decentralised Procedure Omeprazole 10mg Gastro-resistant Capsules, hard Omeprazole 20mg Gastro-resistant Capsules, hard Omeprazole 40mg Gastro-resistant Capsules, hard (UK/H/4423/001-003/DC)
Guidance for Industry
 Guidance for Industry End-of-Phase 2A Meetings U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) September 2009 Procedural Guidance
Guidance for Industry End-of-Phase 2A Meetings U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) September 2009 Procedural Guidance
Biological importance of metabolites. Safety and efficacy aspects
 Biological importance of metabolites Safety and efficacy aspects Bernard Walther Technologie Servier Biological importance of metabolites Safety testing of drug metabolites Bioanalytical strategy Structural
Biological importance of metabolites Safety and efficacy aspects Bernard Walther Technologie Servier Biological importance of metabolites Safety testing of drug metabolites Bioanalytical strategy Structural
Biosimilars: Additional Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009
 Biosimilars: Additional Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009 Guidance for Industry DRAFT GUIDANCE This guidance document is being
Biosimilars: Additional Questions and Answers Regarding Implementation of the Biologics Price Competition and Innovation Act of 2009 Guidance for Industry DRAFT GUIDANCE This guidance document is being
How To Market Pantoprazol Beximco
 Public Assessment Report Scientific discussion Pantoprazol Beximco 20 mg and 40 mg, gastro-resistant tablets (pantoprazole) NL/H/2751/001-002/DC Date: 27 August 2014 This module reflects the scientific
Public Assessment Report Scientific discussion Pantoprazol Beximco 20 mg and 40 mg, gastro-resistant tablets (pantoprazole) NL/H/2751/001-002/DC Date: 27 August 2014 This module reflects the scientific
Guidance for Industry ANDA Submissions Amendments and Easily Correctable Deficiencies Under GDUFA
 Guidance for Industry ANDA Submissions Amendments and Easily Correctable Deficiencies Under GDUFA DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Guidance for Industry ANDA Submissions Amendments and Easily Correctable Deficiencies Under GDUFA DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
THE DIGESTIVE SYSTEM
 THE DIGESTIVE SYSTEM What is digestion? Digestion is the process of breaking down food so that it's small enough to be absorbed and used by the body for energy or in other bodily functions. Digestion involves
THE DIGESTIVE SYSTEM What is digestion? Digestion is the process of breaking down food so that it's small enough to be absorbed and used by the body for energy or in other bodily functions. Digestion involves
Bioavailability / Bioequivalence
 Selection of CROs Selection of a Reference Product Metrics (AUC, C max /t max, Shape of Profile) Acceptance Ranges (0.80 1.25 and beyond) Sample Size Planning (Literature References, Pilot Studies) Steps
Selection of CROs Selection of a Reference Product Metrics (AUC, C max /t max, Shape of Profile) Acceptance Ranges (0.80 1.25 and beyond) Sample Size Planning (Literature References, Pilot Studies) Steps
General Clinical Pharmacology
 General Clinical Pharmacology Considerations for Pediatric Studies for Drugs and Biological Products Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes
General Clinical Pharmacology Considerations for Pediatric Studies for Drugs and Biological Products Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes
A FDA Perspective on Nanomedicine Current Initiatives in the US
 A FDA Perspective on Nanomedicine Current Initiatives in the US Carlos Peña, PhD Office of the Commissioner FDA September 3, 2010 Outline Context Nanotechnology Task Force report summary Identification
A FDA Perspective on Nanomedicine Current Initiatives in the US Carlos Peña, PhD Office of the Commissioner FDA September 3, 2010 Outline Context Nanotechnology Task Force report summary Identification
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
Guidance for Industry Classifying Resubmissions in Response to Action Letters
 Guidance for Industry Classifying Resubmissions in Response to Action Letters U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry Classifying Resubmissions in Response to Action Letters U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
